Diagrama de Lewis del ion fosfato PO4(2) YouTube
PO4 3 Lewis Structure How to Draw the Dot Structure II lSCIENCE ll
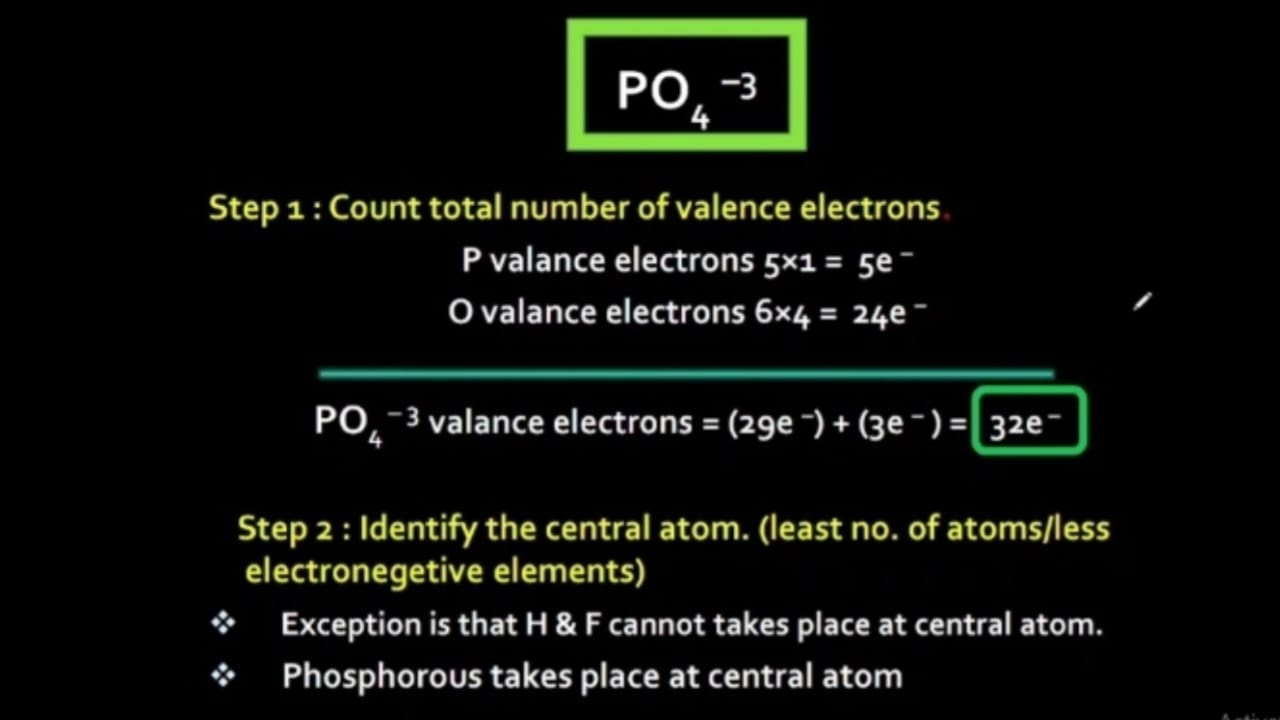
Well, actually, you can draw the Lewis diagram with single bonds, and all the oxygens have 3 lone pairs. PO4^3- has 5+4x6+3 = 32 e-. The only reason that you might want to include a double bond is because of overly strict adherence to formal charges. By making one of the bonds a double bond, you reduce the formal charge on P from +2 to +1 and.

How to Calculate the Formal Charges for PO4 3 (Phosphate ion) YouTube
A step-by-step explanation of how to draw the PO43- Lewis Dot Structure (Phosphate ion). For the PO4 3- structure use the periodic table to find the total number of valence electrons.

Simple Method for writing Lewis Structures of the phosphate ion(PO4)3
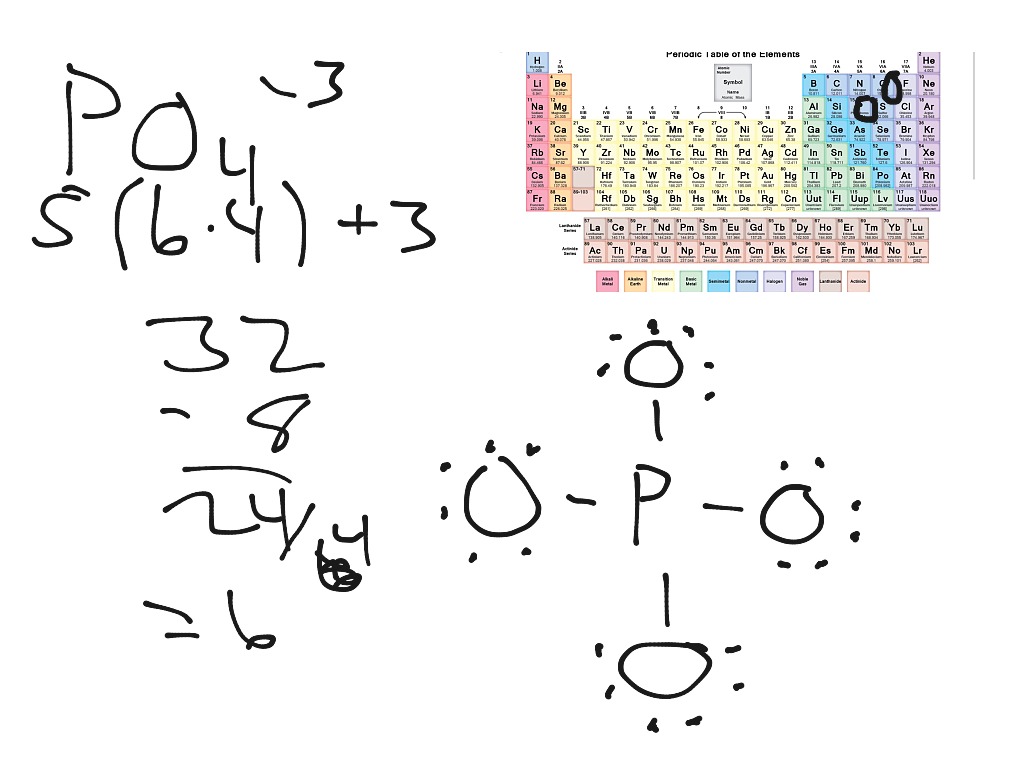
Lewis structure of PO43- ion (phosphate ion) contains one double bond and three single bonds between the Phosphorus (P) atom and Oxygen (O) atoms. The Phosphorus atom (P) is at the center and it is surrounded by 4 Oxygen atoms (O). Let's draw and understand this lewis dot structure step by step.

Draw a single Lewis structure for the phosphate ion (PO4^3−), inwhich
Aurora Chemistry 9.21K subscribers Subscribe Subscribed 3.7K views 3 years ago Lewis structure 01 || Lewis Diagrams In this video, Let us discuss how to write Lewis structure of.

PO4 estructura de lewis urgenteeee Brainly.lat
Share 1.6K views 1 year ago Lewis Structure PO4 3- is a chemical formula for Floroform. It consists of one sulphur atom and four oxygen atoms.
Lewis dot structure for PO4 3 Phosphate ion YouTube
PO4 3- (phosphate ion) lewis structure has a Phosphorus atom (P) at the center which is surrounded by four Oxygen atoms (O). There is 1 double bond and 3 single bonds between the Phosphorus atom (P) and each Oxygen atom (O). There are 2 lone pairs on double bonded Oxygen atom (O) and 3 lone pairs on single bonded Oxygen atom (O).
Lewis structure of PO43 Science, Chemistry, Elements, Atoms
This chemistry video tutorial explains how to draw the lewis structure of PO4 3-, the phosphate ion. It also discusses the formal charge and resonance struc.
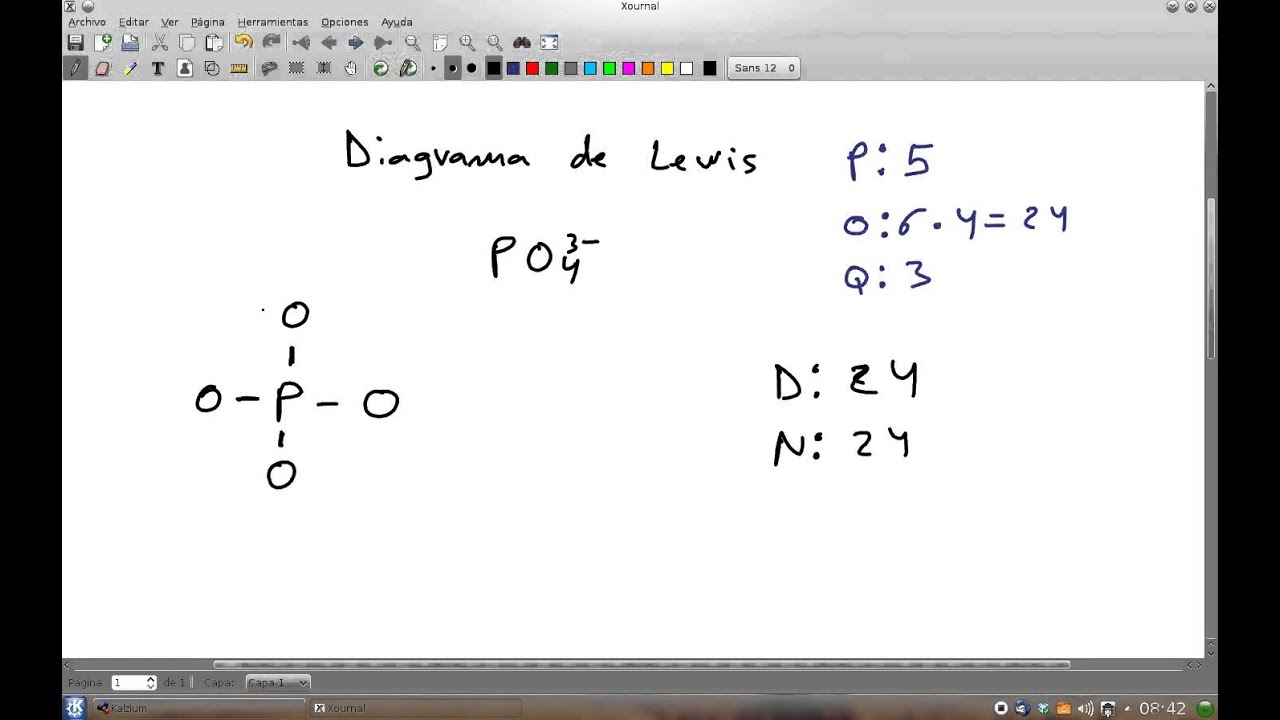
Diagrama de Lewis del ion fosfato PO4(2) YouTube
We start with a valid Lewis structure and then follow these general rules..more.more There are three resonance structures PO43- (Phosphate ion). We start with a valid Lewis structure.

Resonance Structures for PO4 3 (Phosphate ion) YouTube
You can easily draw the Lewis dot structure of a phosphate ion with us using the simple guidelines given below. Steps for drawing the Lewis dot structure of [PO4]3- 1. Count the total valence electrons in [PO4]3- The Lewis dot structure of a molecule is referred to as a simplified representation of all the valence electrons present in it.

Easy steps to draw LEWIS structure of PO4 3 (Phosphate ion) YouTube
Let's do the Lewis structure for PO4 3-. Phosphorus has 5 valence electrons. Oxygen has 6, we've got 4 Oxygens. This negative 3 up here means we have three additional electrons. Five plus 24 plus 3 gives you 32. So those are our valence electrons. Put Phosphorus at the center and the Oxygens around it, all 4 of them.

PO4 3 Lewis Structure How to Draw the Lewis Structure for PO4 3
Step 4 in determining the Lewis Structure of PO4(3-) PO 4 3-Step 4 No Change from step 3 as we have no electrons left over. Picture so Far: Total Valence Electrons: 32: Used so Far: 4(2)+4(6) = 32: Remaining: 0: Last updated: August 27, 2005
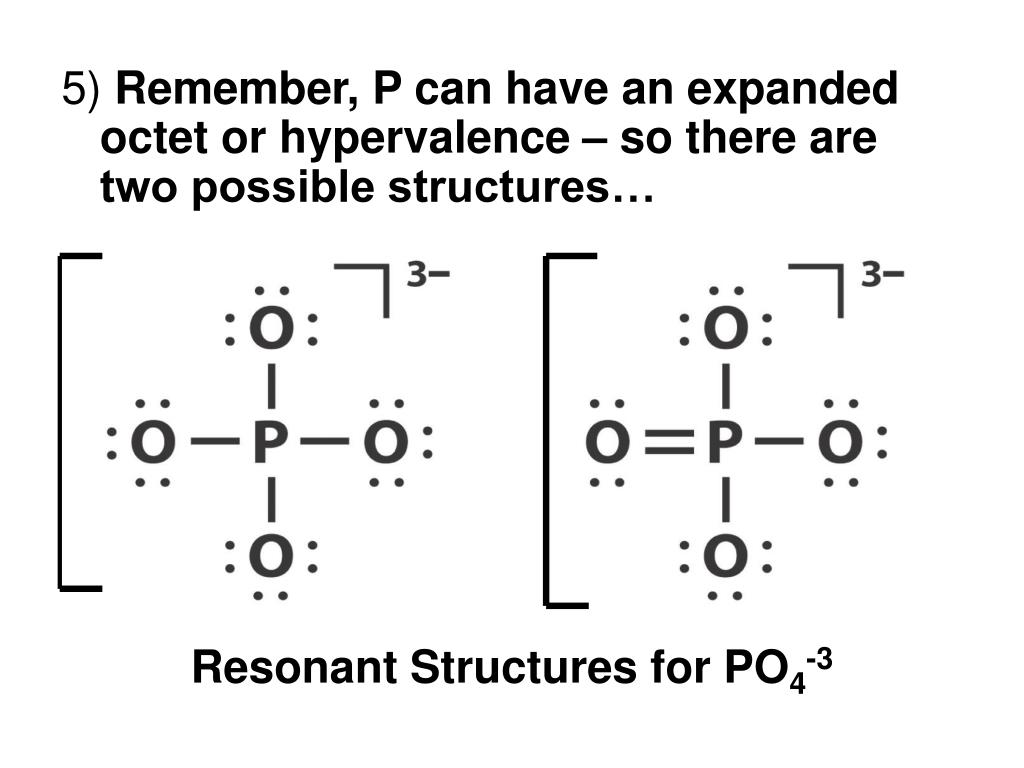
PPT Molecular Bonds Part II PowerPoint Presentation, free download
The Lewis Dot Structure for PO 4 3-: The phosphate anion (PO 4 3-) represents the fully ionized form of phosphoric acid (H 3 PO 4 ). This anion plays significant biochemical roles in living organisms. The Lewis structure can be drawn based on several general rules, and shows how valance electrons are used in the bonding between atoms.
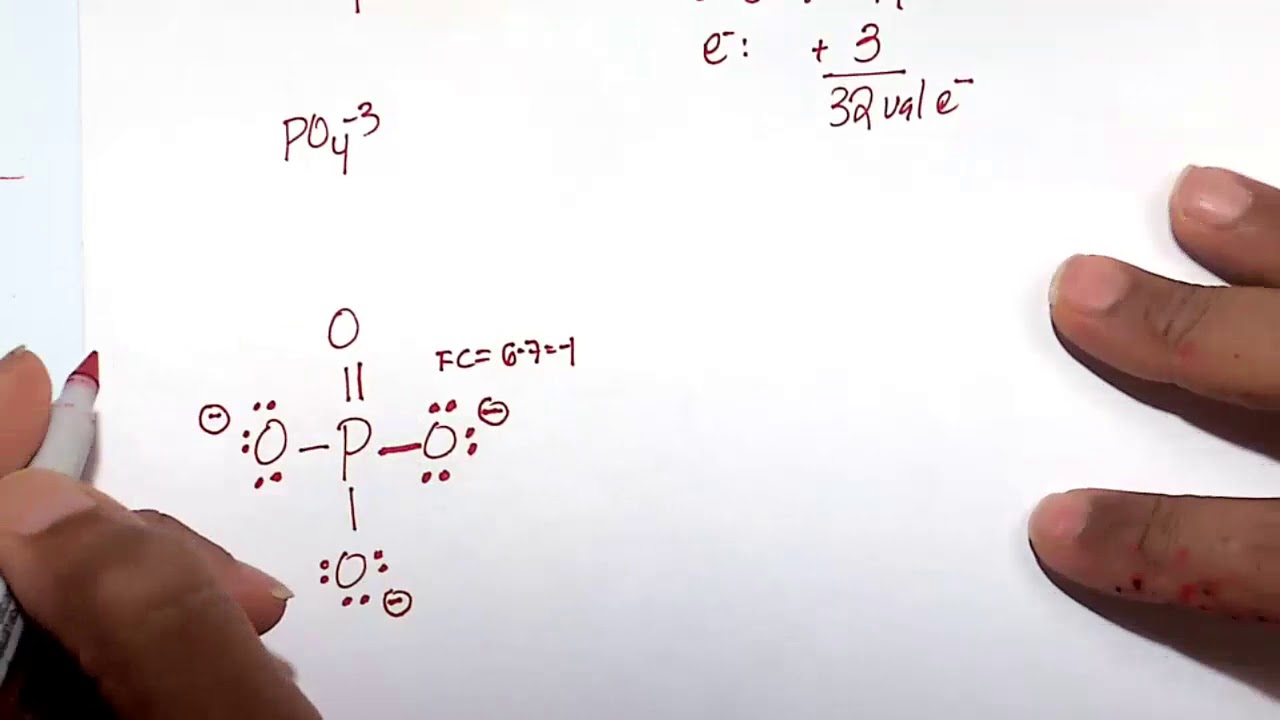
Resonance The resonance structure of the phosphate ion (PO4(3)) YouTube
In the Lewis structure of PO43-, P forms single bonds with 3 oxygen atoms and forms a double bond with one oxygen atom. These oxygen atoms carry a charge of -1. Let us now look that steps required for drawing a Lewis structure:- 1. Counting the total number of valence electrons of the molecule. 2. Locating the central atom of the molecule. 3.

Lewis Dot Structure of Phosphate (PO4 3).....No More Confusion
How to Draw Lewis Structure of PO4 3- I Easy & Quick Science Genius 26 subscribers Subscribe 0 2 views 9 months ago This video will explain how to draw a skeletal structure of phosphate ion..
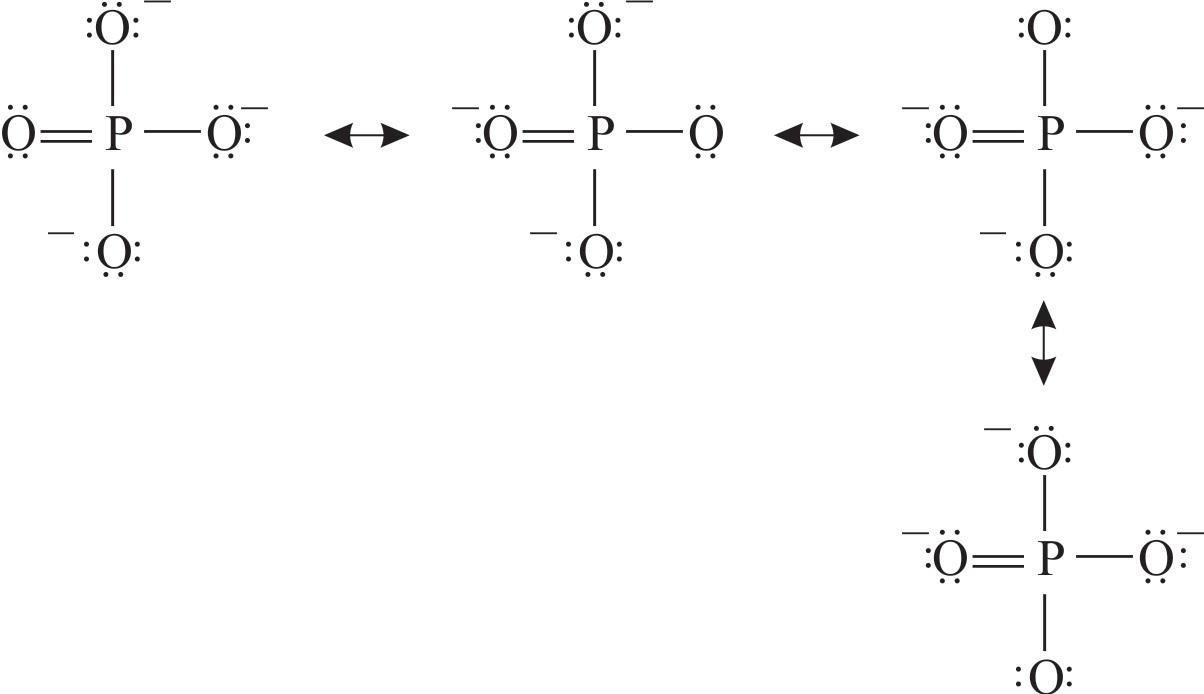
Identify The Lewis Structure Resonance Forms For Po That My XXX Hot Girl
For the Lewis structure for PO4 3- you should take formal charges into account to find the best Lewis structure for the molecule. Remember, PO4 3- has a negative three charge on the molecule. For the Lewis structure you'll need to have a total charge for the molecule of 3-. It is helpful if you:
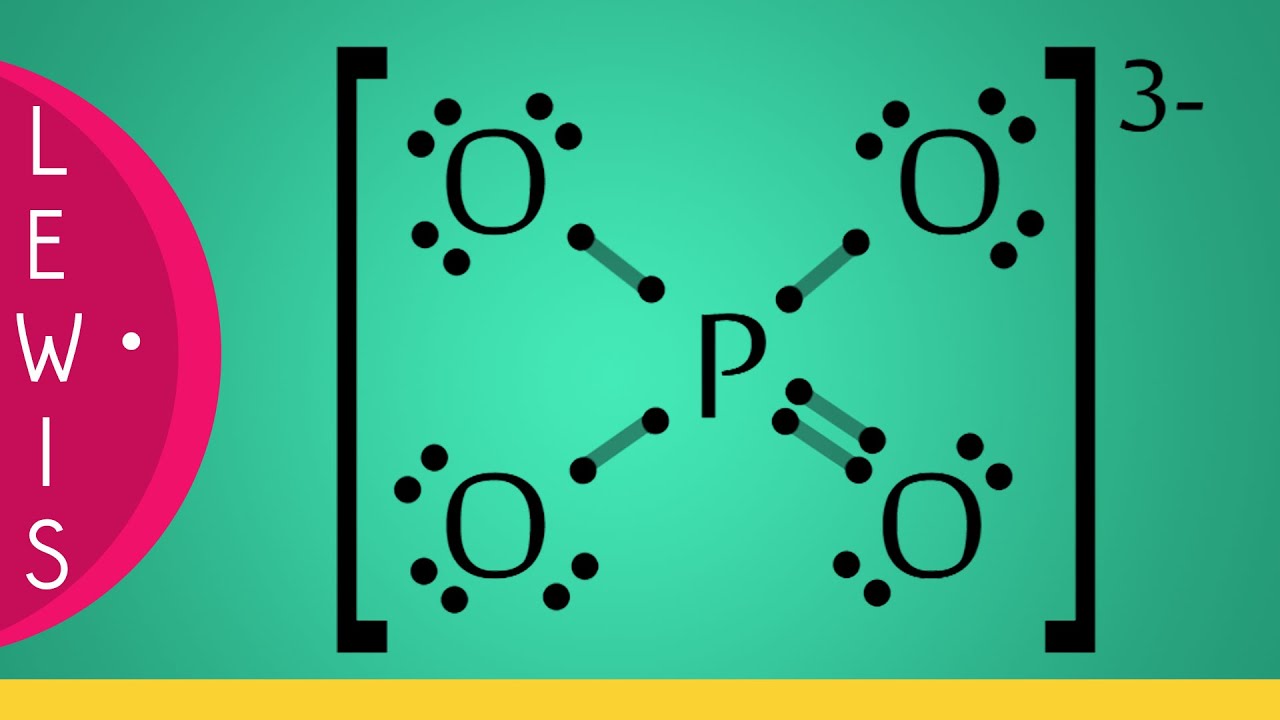
Strutture di Lewis • Ione Fosfato [PO4]3 YouTube
In the lewis structure of PO 43-, three is a double bond between phosphorous atom and one oxygen atom. Between other oxygen atoms, there are only single bonds with phosphorous atom. Also, each oxygen atom has a -1 charge. Related lewis structures to H 3 PO 4 H 3 PO 2 lewis structure H 3 PO 3 lewis structure